CORRESPONDENCE
Isabel María Méndez Sánchez
Costa del Sol University Hospital. Marbella. Malaga.
29603 Marbella, Málaga
CITE THIS WORK
Méndez Sánchez IM, Pereda Salguero T. Eosinophilic gastroenteritis.RAPD 2025;48(1):18-26. DOI: 10.37352/2025481.2
Introduction
Eosinophilic gastroenteritis (EG) or better known as Eosinophilic Gastrointestinal Disease (EGE), as EG is part of this entity, but sometimes we confuse terms, is an uncommon disease, with an estimated prevalence of around 6-8/100,000 inhabitants[1], although other studies estimate a higher prevalence of 30/100,000 inhabitants[2]. It was first described by Kaijser in 1937[3], and can affect both children (more common) and adults, more so in males. When it affects adults, it is most frequent between the third and fifth decade of life[4],[5]. It is characterised by an eosinophilic infiltrate that can involve the different layers of the wall of the gastrointestinal tract, with no secondary cause of eosinophilia[6]. Its origin is unknown and the pathogenic mechanisms have not yet been elucidated, so an allergic cause has been postulated because a variable number of patients have been found to have a personal and/or family history of atopy (50%). There are also other associated risk factors such as higher socioeconomic status, Caucasian race, overweight and a hereditary component[7]. The clinical manifestations depend on the layer of the gastrointestinal tract that is predominantly affected[8].
Etiopathogenesis
Its origin is unknown. Existing studies suggest an allergic component associated with high levels of immunoglobulin E (20-50%)[9],[10]. A Th2 lymphocyte population expressing interleukin 5 has been identified, suggesting that exposure to certain foods activates their differentiation and leads to eosinophilia. Eotaxin has also been shown to increase eosinophil recruitment at the gastrointestinal level in response to food antigen[11], leading to local inflammation by release of cationic cytotoxic proteins from these eosinophils. The infiltration of eosinophils into tissue and their degranulation leads to tissue fibrosis, thrombosis, small vessel vasculitis and persistent inflammation[12]. However, the pathogenic hypothesis of allergy cannot be sustained in all cases, as the personal or family history of allergic disease is not constant.
The location may be in any part of the gastrointestinal tract, with a predilection for the disease in the stomach and small intestine[13]. If the stomach is exclusively affected, it is called eosinophilic gastritis (EG), the stomach and small intestine: eosinophilic gastroenteritis (EG), the small intestine: eosinophilic enteritis (EE) and the colon: eosinophilic colitis (EC), although there may be diffuse involvement[14].
Clinical
The clinical manifestations of EGE are very varied and depend on the predominantly affected layer, location and extent of the disease[15],[16], according to Klein's classification.
Mucosal infiltration (the most common) manifests with abdominal pain, diarrhoea, nausea, vomiting, weight loss and malabsorption (the latter especially in those with diffuse disease)[17]; muscular infiltration, the second most frequent, can cause abdominal pain secondary to subocclusive or obstructive symptoms if it affects the stomach, colon or small intestine, pseudochalasia if it affects the oesophagus, sometimes even perforation of the affected gastrointestinal tract[18] and if serosal infiltration predominates, the rarest involvement, in the form of eosinophilic ascites8 . There are also other reported cases of complications such as eosinophilic cystitis (4.5%)[19], acute pancreatitis 20 and liver dysfunction[21] .
In the article published by the group of Abou Rached A et al[22], they suggested dividing the disease into four classifications: mild, moderate, severe and complicated, based on the initial clinical manifestations, laboratory, radiological, endoscopic and histological findings.
Diagosis
The diagnosis of this entity is established on the basis of these four criteria[8]:
Gastrointestinal symptoms suggestive of EGE.
Demonstration of eosinophilic infiltration in one or more areas of the gastrointestinal tract on biopsy (note that mucosal biopsies are normal in subjects with disease localised in the muscularis and serosal layer).
Absence of eosinophilic involvement of extradigestive organs.
Absence of parasitic infestation - eosinophilia-inducing drugs -malignancy
Laboratory tests
Peripheral eosinophilia occurs in 80% of patients, more frequently if it affects the mucosal and serosal layer[4]. Furthermore, there is no relationship between the extent of peripheral eosinophilia and the degree of tissue infiltration or epithelial damage[10]. In addition, altered D-xylose test, anaemia, positive TSOH, elevated IgE especially in children, elevated ESR (25%) can also be observed. High levels of faecal and serum eosinophilic cationic eosinophilic protein have also been reported.
Imaging tests
In most patients radiological findings are normal[23]. Barium tests, CT and MRI reveal thickening of the intestinal wall or saw-tooth appearance of the small bowel mucosa. Ascites may also be seen.
Endoscopy
In half of the patients the endoscopic examination is normal (although in the article published by Hui et al in 2018[24] describe 92% of normal colonoscopies). When findings are present they are usually non-specific: erythema, nodular mucosa, polyps, erosions and deep ulcers. It is recommended to take multiple biopsies, given the patchy involvement of the disease, at least 5 from normal areas of the stomach and small intestine, and to take biopsies from abnormal areas. If the biopsies are negative, this does not rule out EGE, because the involvement may be muscular or serous, and it is recommended that a full-thickness biopsy be performed by laparoscopy or endoscopy (full-thickness), especially in those who debut with intestinal obstruction and have thickening of the wall. Endoscopic biopsies with high-capacity forceps or endoscopic resection of the suspicious mucosa could also be attempted[14]. Echoendoscopy can also play an important role in cases of muscular and serosal involvement and facilitates biopsy by FNA[25].
Food allergy tests (allergen-specific IgE and prick test).
There are many doubts as to their clinical application in this entity. These tests lack both sensitivity (they miss about 40% of the causative agents) and specificity, so they do not effectively identify the foods that cause this condition, and even if they are suppressed in many cases, there is no improvement.
Anatomical Pathology
Histological definition
EGE is a group of processes pathologically characterised by excessive eosinophils in mucosal biopsies from single or multiple sites of the gastrointestinal tract (GIT), simultaneously or sequentially, in the absence of secondary tissue eosinophilia. They are subclassified according to the site affected as eosinophilic oesophagitis (EE), eosinophilic gastritis, eosinophilic enteritis, and eosinophilic colitis.
Eosinophils are leukocytes with a bilobed nucleus and cytoplasm rich in fine pink granules with eosin staining, to which they owe their name coined by Ehrlich in 1879[26]. They are normally present in the mucosa of the entire gastrointestinal tract, with the exception of the oesophagus, where their presence is always pathological, but there are few studies that quantify the normal number of eosinophils, which complicates the ability to recognise a pathological increase in their number[27]. This difficulty also lies in the high intra- and interpersonal variability of normal eosinophil numbers, which is influenced by age, with numbers graded upwards along the GIT. The diagnosis may also be influenced by the representativeness of the endoscopic biopsy, either because it may present a patchy distribution, or involve one or more layers of the wall of the affected gastrointestinal segment. It may also depend on the subjectivity of the pathologist and even the variability of high power field sizes (HPF) according to the different microscopes used. There is a historical lack of diagnostic criteria and standardised methodology for eosinophil counts. There are currently no consensus recommendations except for eosinophilic oesophagitis[28], although concepts and terminology are recently being reviewed[29].
The most practical way to report the number of eosinophils in current practice would be a maximum peak in the most inflamed HPF, rather than multiple field counts and averaging, which is preferable for research studies[27].
Pending formal diagnostic criteria, the following cut-off points have recently been suggested for the number of eosinophils per HPF required for the diagnosis of EGE according to the location in the GIT:[30],[31]
-Stomach: ≥30 eosinophils per HPF in 5 HPFs.
-Duodenum: ≥52 eosinophils per HPF.
-Ileum: >56 eosinophils per HPF
-Right colon: >100 eosinophils per HPF
-Transverse and descending colon: >84 eosinophils per HPF.
Given that eosinophils are a normal component of the gastrointestinal mucosa and the great variability in their numbers that they can present, establishing a diagnosis of eosinophilic gastroenteritis in endoscopic biopsies can be problematic. Collins proposed to differentiate between mucosal eosinophilia, when there is a slight increase in the number of eosinophils without additional pathological changes. The designation EGE would be reserved, when in addition to tissue eosinophilia there are additional pathological changes such as eosinophil degranulation, presence of intraepithelial eosinophils (superficial or cryptic), cryptic eosinophilic abscesses, architectural changes, and presence of eosinophils in mucosal muscle, submucosa or both[27]. This approach would avoid overdiagnosis of these processes that could lead to inappropriate treatment. On the other hand, due to the possible patchy distribution of these processes, along the GIT and depending on the wall layer affected, they may be underdiagnosed in endoscopic biopsies. It is advisable to take multiple biopsies, at least 4-5 from each location, of normal and abnormal mucosa. If the initial endoscopic mucosal biopsy is normal or mildly changed and suspicion of EGE persists, repeat biopsies may be indicated.
In general, features commonly found in EGE biopsies include:[30]
-Increased numbers of eosinophils. An extreme numerical increase (>100 per HPF) by itself may merit a diagnosis of EGE.
- Altered distribution of eosinophils. In normal gastrointestinal biopsies, eosinophils appear as discrete cells spread evenly in the deep lamina propria. In EGE biopsies, eosinophils appear as sheets in the lamina propria and eosinophils may be found in abnormal locations. These alterations should be considered significant for eosinophil-related disease. Small numbers of intraepithelial eosinophils are normal but markedly increased numbers of intraepithelial eosinophils, or eosinophil abscesses from glands or crypts, may indicate disease even in the absence of excess eosinophils in the lamina propria.
-Pathological alterations related to eosinophils, such as reactive epithelial changes (reduced mucin, increased mitotic activity, etc.).
- Absence of acute inflammation. The presence of acute inflammation in small bowel and colon biopsies that also show prominent eosinophil inflammation should be a reason to consider inflammatory bowel disease. Numerous eosinophils in colon biopsies may portend a poor prognosis in ulcerative colitis.
Eosinophilic gastritis (EG)
In the stomach, a mean eosinophil density greater than or equal to 127/mm (30 or more eosinophils per high power field in at least 5 separate fields) is required for diagnosis[32]. The most common histopathological findings are the presence of eosinophil sheets expanding the lamina propria in more than 50% of cases with altered behaviour and distribution of eosinophils, which tend to surround the foveola with the presence of intraepithelial eosinophils, although they typically do not extend into the lumen forming abscesses. Extension to the mucosal muscle or into the submucosa and degranulation of eosinophilic cryptitis/abscesses is observed in the absence of significant acute or chronic inflammation. They are often accompanied by regenerative epithelial changes and foveolar hyperplasia, or even necrosis and degeneration, but rarely develop frank ulcers. The differential diagnosis of primary or idiopathic GE includes several known causes of tissue eosinophilia in the stomach such as infections (H. Pylori, CMV), parasites (Anisakis spp., Strongyloides stercoralis), drugs, NSAIDs and after eradication treatment for H. Pylori, among others, food allergies, inflammatory bowel disease, vasculitis, connective tissue diseases, and solid and haematological malignancies[32]. It is of interest to know that drugs do not usually cause severe gastric eosinophilia as well as to consider invasive adenocarcinoma in the differential diagnosis. While intestinal-type adenocarcinoma is usually infiltrated by neutrophils, diffuse and signet ring adenocarcinoma frequently recruit eosinophils[33].
Eosinophilic enteritis (EE)
There is no consensus on normal ranges of eosinophil numbers in different segments of the small intestine. Low counts may be observed in normal biopsies (allergy or hypersensitivity). Pure eosinophilic duodenitis is not described and is exceptional. Sampling of duodenum, first cm of jejunum, and terminal ileum is recommended. The cut-off points for the number of eosinophils per HPF required for diagnosis are ≥ 30-52/ HPF in duodenum and >56 / HPF in ileum[5]. Apart from the numerical increase, altered behaviour and distribution of eosinophils infiltrating the surface epithelium is observed with counts of >2 Eo/HPF in duodenum or >4 in ileum. In cryptic epithelium, >6 Eo/HPF in duodenum and >4 in ileum. Other histological findings are the presence of eosinophils in muscularis mucosae and submucosa, eosinophilic cryptic abscesses, extensive degranulation, reactive epithelial changes, villous atrophy and crypt hyperplasia, epithelial cell necrosis, mast cell infiltrate or mesenteric lymph node hyperplasia due to eosinophilic infiltration with minimal chronic and active inflammation. For a diagnosis of EE, which is less common, the usual secondary causes of eosinophilia such as drugs, food allergies, parasites, inflammatory bowel disease, connective tissue disease/vasculitis, and neoplasms, as well as systemic eosinophilic disorders (idiopathic hypereosinophilic syndrome, chronic eosinophilic leukaemia, systemic mastocytosis) should be excluded[26].
Eosinophilic colitis (EC)
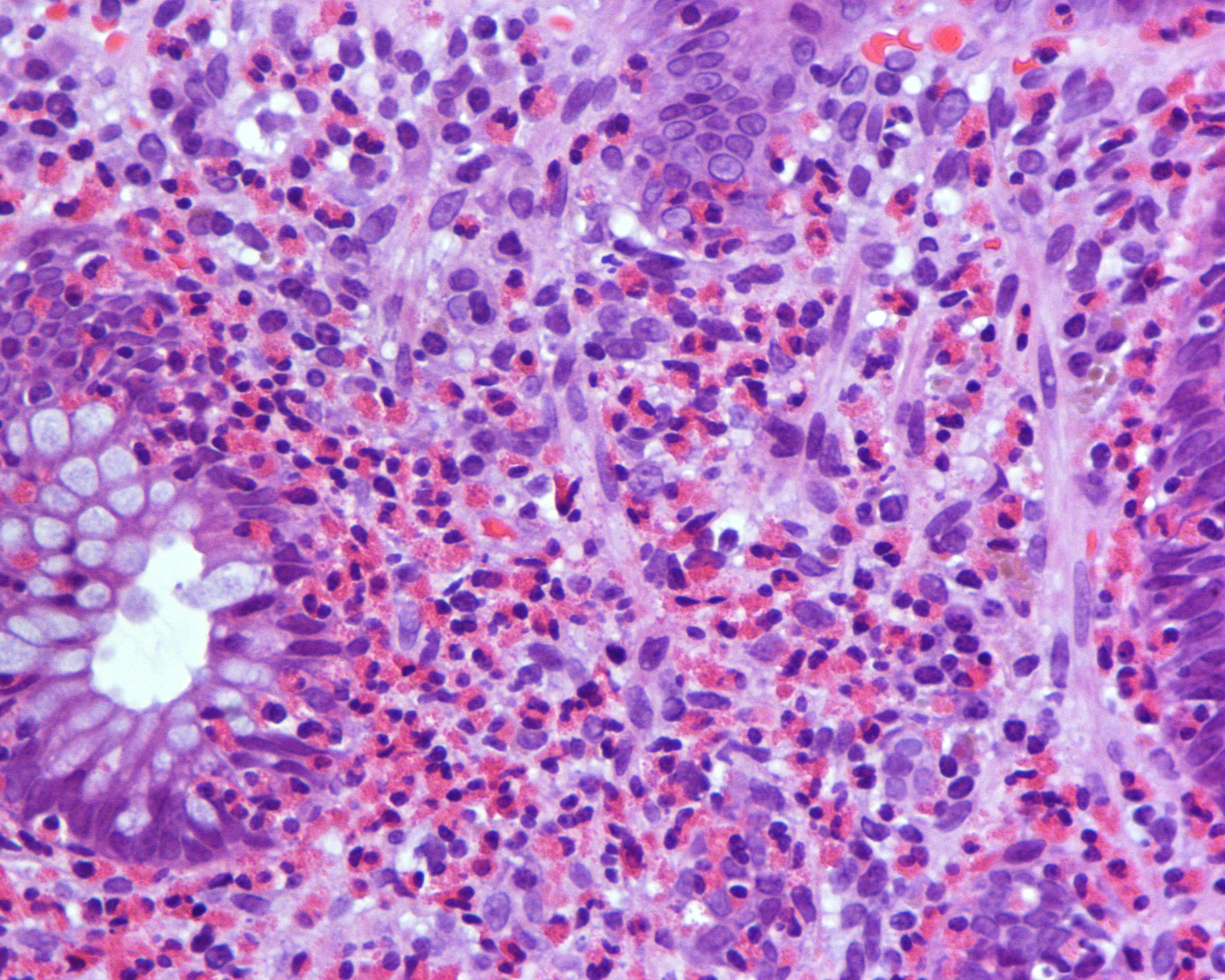
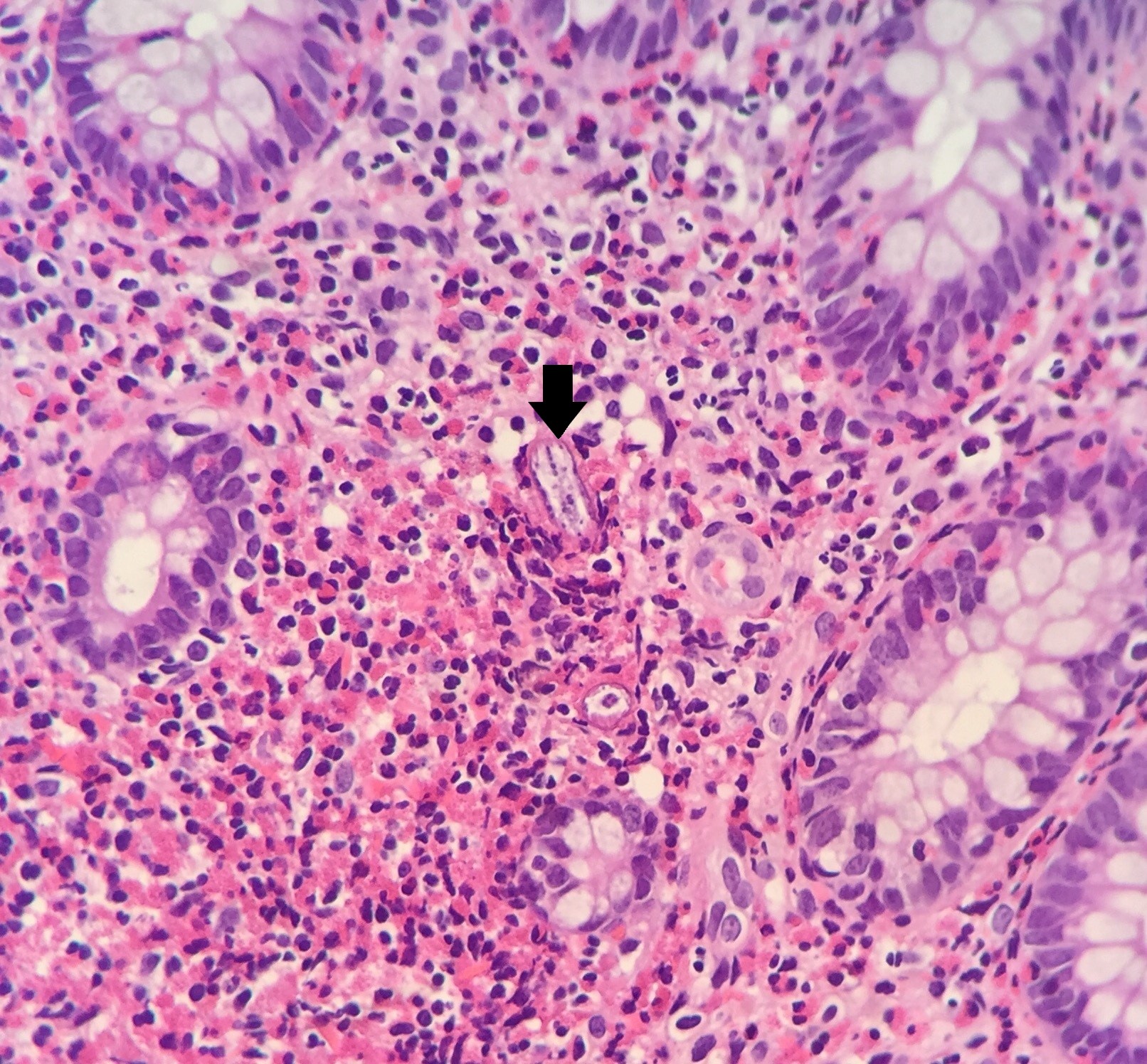
Eosinophil counts in normal colonic mucosa range from 10-70/HPF. There is no universally accepted range for diagnosing CE, more than 2 times the normal number. As mentioned above, cut-off points for the number of eosinophils per high power field required for diagnosis have been suggested, which show a decreasing gradient along the colon[30],[31]. As in the other locations, there is also an altered behaviour and distribution of eosinophils, which excessively infiltrate the superficial epithelium, with the presence of >3 Eo/HPF in the right colon, >4 in the transverse and descending colon and >2 in the rectosigmoid colon. Cryptitis (Figure 1) and cryptic eosinophilic and eosinophilic cryptic abscesses in cryptic epithelium are observed: >11 Eo/HPF in CD; >4 in CT/CD; >9 in rectosigma and extension to muscularis mucosae and submucosa, as always in the absence of significant acute or chronic inflammation. The causes of secondary eosinophilia to rule out are the same as for EE. Parasites can provoke an intense eosinophilic response because this finding should prompt a search for helminth larvae such as S. stercoralis (Figura 2) , Schistosoma eggs or fragments of Trichuris trichiura, with serial sections.
Differential diagnosis
The differential diagnosis should be made with those pathologies that present with blood and/or tissue eosinophilia associated with gastrointestinal symptoms. Therefore, the possibility of : Intestinal parasitosis (Ancylostoma, Anisakis, Ascaris, Strongyloides, Toxocara, Trichiura, Capillaria, Basidiobolomycosis, Trichinella), taking drugs (gold salts, azathioprine, cotrimoxazole, carbamazol, carbamazepine, NSAIDs), malignancy (lymphoma, leukaemia, gastric cancer and colorectal cancer), mastocytosis (a rare disease characterised by abnormal proliferation and accumulation of mast cells in various organs and systems. Clinically heterogeneous, it ranges from occasional pruritus to severe and repeated episodes with life-threatening mediator release. It frequently presents with eosinophilia), inflammatory bowel disease (especially Crohn's disease (tissue eosinophilia is present, rare peripheral eosinophilia), hypereosinophilic syndrome, polyarteritis nodosa, eosinophilic granulomatosis with polyangiitis and eosinophilic granuloma[34].
Treatment
Treatment is based on the little evidence available and on the severity of the disease, and there are no prospective controlled clinical trials.
-First line of treatment: Dietary treatment. If a limited number of allergens are detected in allergy testing, a targeted elimination diet is recommended[5]. If many or none are identified, it is recommended to start an elemental or empirical elimination diet eliminating the six most common food allergens (soy, wheat, egg, milk, nuts, fish/shellfish) for 6 weeks[35] . Fewer foods may be tried in mild disease. The main drawback of this type of diet is patient adherence, so it should be implemented in those who are motivated and under the guidance of an expert nutritionist. If a history of environmental allergens is identified, these should also be eliminated. Follow-up of these patients is based on symptomatology and changes in peripheral eosinophilia within four to six weeks. In patients with peripheral eosinophilia, a >50% reduction in eosinophils can be considered a good response to treatment. However, this follow-up option is not valid in patients with no peripheral eosinophilia or in those with other associated allergic diseases that also generate peripheral eosinophilia and do not respond to dietary treatment. There may also be a poor correlation between symptoms and histology, so it is important to perform biopsies of the gastrointestinal tract if in doubt. If there is a response after the diet, foods can be slowly reintroduced from the least allergenic to the most allergenic. In a study published in 2009[36], three of seven adults on an empirical elimination diet and all six adults on an elemental diet had significant symptom reduction, complete histological remission, endoscopic improvement and normalisation of peripheral eosinophilia within six weeks. Another prospective study in 2020, involving 15 adults, showed that after six weeks on the elemental diet, histological remission rates were 100%[37].
-2nd line of treatment: Corticosteroids. If there is no improvement with the elimination diet/elemental diet, or the patient refuses it, it is recommended to start treatment with steroids. Most of the reported case series have shown a response rate of up to 90%[38], although in more current reviews the values are lower (50%)[39]. Among patients with EGE, those with a predominantly serous pattern seem to respond best[40]. Regarding dosage, it is recommended to start at 20-40 mg/day (although in some series higher doses of 0.5-1 mg/kg/day have been suggested)[41]. Response usually occurs within two weeks of starting treatment, regardless of the affected layer, although others require longer treatment to control symptoms, so maintenance with induction doses for 2-6 weeks is recommended. Subsequently, we start a progressive reduction of the dose gradually, from weeks to months. However, some patients (25-70%) relapse on dose reduction, requiring prolonged treatment with a minimum dose. The aim is to be able to use the lowest dose necessary to control the symptoms of EGE. If there is no response to the oral route, the intravenous route should be used. Budesonide would also be an alternative in patients with proximal and distal disease (dissolved in water or crushed[42] ) with the advantage of fewer side effects due to its lower systemic impact.
-Other alternatives: Evidence for other drugs is based on isolated cases or small case series, and many in clinical trials not yet completed.
· Azathioprine: Immunosuppressive agent. Its efficacy has been demonstrated in patients with refractory and steroid-dependent disease[43]. The dose is not well established due to the lack of controlled clinical trials, although it is usually used at the same dose as in inflammatory bowel disease (1.5 to 2.5 mg/kg/day), with prior measurement of TPMT enzyme levels. Other immunosuppressants such as 6-Mercaptopurine and tacrolimus may also be used.
· Mesalazine at doses of 2-4g per day.
· Mast cell inhibitors such as Sodium cromoglycate 800 mg per day, Ketotifen starting at 1 mg nightly with increments of 2-4 mg/day, for one to four months.
· Leukotriene antagonist: Montelukast at a dose of 10-40mg/day.
· Humanized anti-IL-5 antibody such as Mepolizumab: in one study it was shown to induce response in a group of six patients with EG, although subsequent relapse in eosinophilia was demonstrated in all responders with associated clinical relapse[44] ; Benralizumab: current phase 3 clinical trial, where the effect will be evaluated at 24 weeks; Reslizumab : in a pilot study, in which an intravenous dose (1 mg/kg) was administered, it was shown to be effective in reducing tissue and peripheral eosinophilia despite failure of symptomatic control[45]. On the other hand, Kim et al.[46] demonstrated improvement of eosinophilia and symptoms in six of eight patients with EG. However, recurrence of eosinophilia was the norm after discontinuation of the drug which, according to the authors, could be secondary to IL-5, since it was reversed with in vitro administration of Reslizumab.
· Anti-IgE monoclonal antibody (Omalizumab)[34]. In an article including nine patients, it significantly improved symptom scores and decreased gastroduodenal eosinophil count[47]. Randomized controlled studies are required to clarify its efficacy and safety in the management of this type of patient.
· Anti IL4/IL13: Dupilumab has been certified to reduce symptoms and eosinophilic infiltration in eosinophilic esophagitis. With respect to EGE, clinical trials are ongoing to prove its efficacy.
· Fecal transplantation : In an article published by the group of Dai et al[48], therapeutic efficacy was observed in a patient with EG who had undergone fecal transplantation and prednisone treatment. It is still unclear whether fecal transplantation could cure EG or maintain long-term clinical remission.
· Surgical treatment: Surgery may be necessary when complications occur or when the disease presents as an acute abdomen (obstruction or perforation). However, it should be avoided whenever possible, as it is not curative.
Natural History
The natural history is unknown, because the existing series of patients are small. Generally, EGE has a good prognosis, since mortality is rare except for complications associated with the process, and there does not seem to be an increased risk of development of neoplasms. Three forms of evolution are postulated:
-After the outbreak, spontaneous remission.
-Others present exacerbation episodes months or years after the first episode.
-After the first outbreak, chronic course.
In an article published by a French group[49] selected a total of 43 patients, with a mean follow-up of 13 years, identifying three possible courses of the disease: 42% remitted completely, 37% had periods of exacerbation and 21% presented a chronic disease after the first exacerbation. They also concluded that the serous pattern has a relatively good prognosis presenting a majority of single flare-ups and no chronic course. On the contrary, the mucosal predominant pattern presented mostly a chronic continuous course and the muscular pattern was the most prone to relapse. Untreated patients may progress to malabsorption and severe malnutrition[50] and others to intestinal obstruction and perforation.

 Descargar número completo
Descargar número completo Download full issue
Download full issue